AYUSH Ministry asks Patanjali to stop advertising its anti-COVID-19 drug 'Coronil'
Total Views |
New Delhi, June 24: Following Baba Ramdev-led Patanjali launched the first Ayurvedic cure for COVID-19 'Coronil and Swasari', the Union Ministry of Ayush issued a statement asking the company to stop advertising. It further stated that publishing such claims as the facts of the claim and details of the stated scientific study are not known to the Ministry on Tuesday.
"The concerned Ayurvedic drug manufacturing company has been informed that such advertisements of drugs including Ayurvedic medicines are regulated under the provisions of Drugs and Magic Remedies (Objectionable Advertisements) Act, 1954 and Rules thereunder and the directives issued by the Central government in the wake of COVID outbreak," the statement read.
To verify the claims made by Patanjali Ayurved, the Ayush Ministry asked the firm to provide at the earliest "details of the name and composition of the medicines being claimed for COVID treatment; site(s)/hospital(s), where the research study was conducted for COVID-19; protocol, sample size, Institutional Ethics Committee clearance, CTRI registration and results from data of the study (ies) and stop advertising/publicizing such claims till the issue is duly examined."
The Ministry also has requested concerned State Licensing Authority of Uttarakhand government to provide copies of license and product approval details of the Ayurvedic medicines being claimed as treatment of COVID-19. Meanwhile, after the Ministry issued the statement Patanjali CEO Acharya Balakrishnan has stated that the company has provided the information as demanded.
Acharya Balkrishna remarked, 'Communication gap has been done away with and we have 100% fulfilled all standard parameters for Randomised Placebo-Controlled Clinical Trials'. He also heaped praise on the ruling government for providing 'encouragement and pride' to Ayurveda.
यह सरकार आयुर्वेद को प्रोत्साहन व गौरव देने वाली है जो communication gap था वह दूर हो गया है व Randomised Placebo Controlled Clinical Trials के जितने भी Standard Parameters हैं उन सबको 100% fullfill किया है इसकी सारी जानकारी हमने आयुष मंत्रालय को दे दी है @moayush @yogrishiramdev pic.twitter.com/0CAMPZ3xvR
— Acharya Balkrishna (@Ach_Balkrishna) June 23, 2020
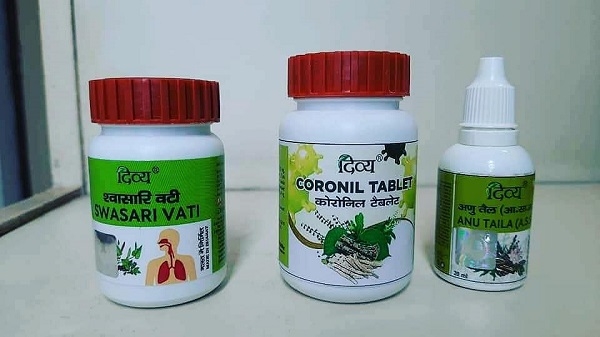
Patanjali Ayurved on Tuesday launched 'Ayurved-based medicines Coronil and Swasari' claiming that it has shown a 100 percent efficacy against coronavirus infection within 3-7 days. He claimed that the entire kit would help in strengthening the immunity of a person. According to Baba Ramdev, active compounds of Ashwagandha, Giloy, and Tulsi had been used in 'Coronil'. The clinical trials have been stated to be jointly done by Patanjali Research Institute and NIMS University, Jaipur.
Patanjali's anti-COVID kit includes 3 medicines Coronil tablet, Anu Taila, and Swasari Vati. The COVID-19 therapy suggested by Pantanjali is applicable for adults ranging between 15-80 years of age whereas children are advised to take half the dosage prescribed for adults.


